
Initially, there was no theoretical explanation for the Periodic Law, and it was only used as an empirical principle however, with the development of quantum mechanics, it became possible to understand the theoretical basis for the Periodic Law. The periodic trends tell you in which direction of the periodic table do you have increasing values for different chemical properties. Lothar Meyer presented his table following Mendeleev, but he disagreed with Mendeleev’s Periodic law.
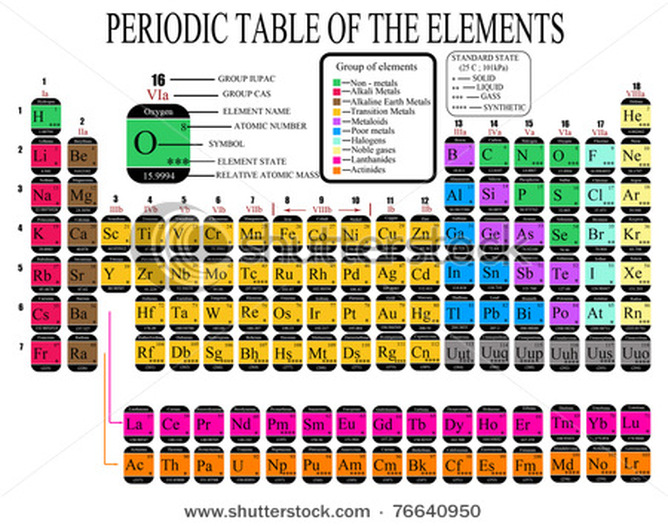
Henry Moseley discovered in 1913 that periodicity is determined by the atomic number rather than the atomic weight. Mendeleev also proposed an elemental periodic system based not only on atomic weights but also on the chemical and physical properties of the elements and their compounds. Mendeleev discovered this principle in 1871, following a number of investigations throughout the nineteenth century. ISRO CS Syllabus for Scientist/Engineer Exam.ISRO CS Original Papers and Official Keys.GATE CS Original Papers and Official Keys.DevOps Engineering - Planning to Production Periodic trends (such as electronegativity, electron affinity, atomic and ionic radii, and ionization energy) can be understood in terms of Coulomb's law.Python Backend Development with Django(Live) The major periodic trends in the modern periodic table are electronegativity, electron affinity, ionization energy, atomic radius, melting point, and metallic.Android App Development with Kotlin(Live) Covalent or atomic radius increases going down a group of the periodic table as there are more electron shells in atoms.
#Trends in the periodic table chemistry full

Java Programming - Beginner to Advanced.Data Structure & Algorithm-Self Paced(C++/JAVA).Data Structures & Algorithms in JavaScript.Data Structure & Algorithm Classes (Live).


 0 kommentar(er)
0 kommentar(er)
